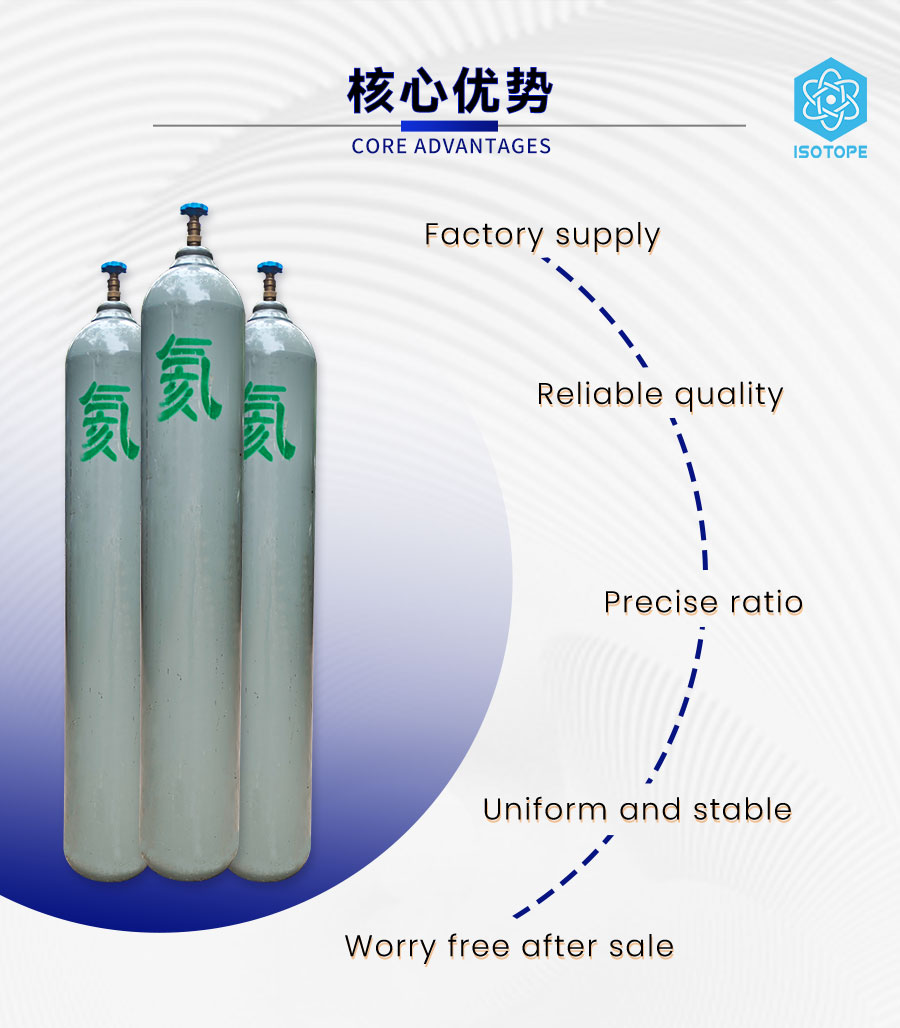
Gasbeskrivning
Helium, även känt som helium med hög renhet eller helium med ultrahög renhet. Det är en ädelgas med inaktiva kemiska egenskaper, och det är i allmänhet svårt att reagera med andra föreningar eller ämnen. Innehållet av helium med ultrahög renhet i luften är 5,2 ppm. Det är teoretiskt möjligt att separera och extrahera helium med ultrahög renhet från luften, men på grund av dess låga innehåll, höga extraktionssvårigheter och höga separationskostnad är heliumresurser med ultrahög renhet ganska knappa.
Förvara helium med ultrahög renhet på en sval och ventilerad plats, borta från gnistor och värmekällor.
Kemisk formel: He
Molekylvikt: 4,0026
Smältpunkt: -272,2 ℃
Kokpunkt: -268,93 ℃
Relativ densitet (vatten=1): 0,15 (-271 ℃)
Relativt ångalösenord: (Air=1): 014
Mättat ångtryck (kpa): 20,64 (-268 ℃)
Kritisk temperatur (℃): -267,9 ℃
Kritiskt tryck (MPa): 0,23
Produktanvändning
Helium med ultrahög renhet används i stor utsträckning inom militär, vetenskaplig forskning, kemi, kylning, medicin, halvledare, rörledningsläckagedetektion, supraledande experiment, metalltillverkning, djuphavsdykning, högprecisionssvetsning och optoelektronisk produktproduktion.
På grund av sin lätta och icke brandfarliga natur kan helium med ultrahög renhet användas för att fylla gasbåtar, ballonger, termometrar, elektroniska rör, dykardräkter och mer.
Helium med ultrahög renhet har låg löslighet i blodet och kan tillsättas till syre för att förhindra tryckfallssjuka. Den kan användas som andningsgas för dykare eller för att behandla astma och asfyxi.
Temperaturen på flytande helium (-268,93 ℃) är nära den absoluta nollpunkten (-273 ℃) och används vanligtvis som en supervätska i supraledande forskning för att tillverka supraledande material.
Flytande helium används också ofta som kylmedel och kylmedel, och inom medicinen används det för argonheliumknivar för att behandla cancer.
Helium med ultrahög renhet (6N) gynnas av universitetslaboratorier, medicinska och forskningsinstitutioner och används ofta i medicinsk och vetenskaplig forskning.